Accelerating the Conformity Assessment process
Mr. Zucker, what is the story behind the creation of MedtechVault?
"As a medical device entrepreneur, I experienced the ordeal of taking a medical device to the market under the MDD regime and I was taken aback by the number of menial tasks, manual ways, and bureaucratic hold-ups that characterized the process. Like many before me, I learned a lot during this time. However, the opportunities to accelerate the process using modern technology became very apparent.
"We chose to target the conformity assessment process. Imagine that your technical documentation is finally completed and you can now submit it to the notified body for assessment. If you are anything like myself, you have a strong feeling of completion and relief at this point. You feel that most of the work is done and you are very close to finally access the market.
Well, lo and behold, now starts a long process of email ping-pong between yourself and the assessor about locating particular pieces of information in the documentation, about not having access to the right documents, and general confusion. Documents are sent back and forth, questions are asked and asked again, long chains of emails are exchanged and there is an increasing feeling of being stuck in a bureaucratic quagmire."
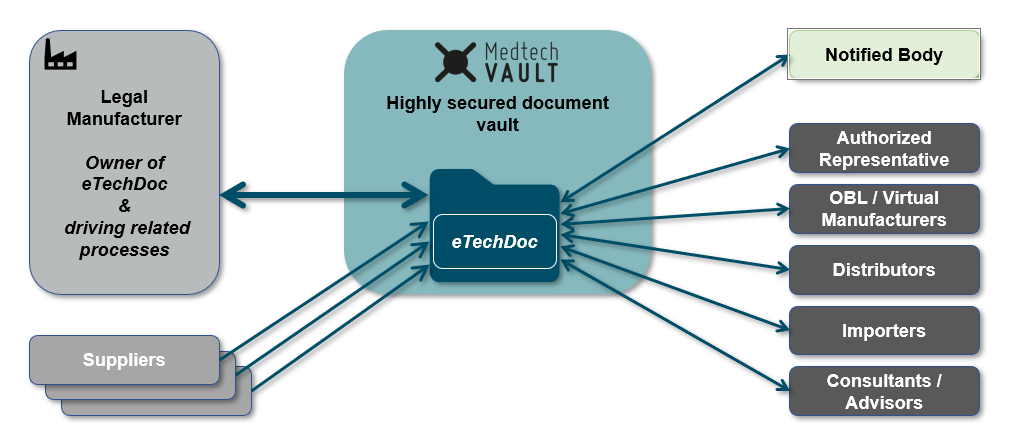
"With MedtechVault, we set out to create a digital collaboration platform for structured and secure communication between the Notified Bodies and the medical device manufacturer. With our current approach, estimations show an (up to) 70% work reduction while simultaneously accelerating the assessment process. Involved stakeholders include the Legal Manufacturer, Critical Suppliers & Notified Bodies / Regulatory Authorities. Of course, any other Economic Operators can be included as well."
With the new playing rules of MDR, I assume that MedtechVault not only concerns manufacturers?
"Exactly. As you know, MDR has had massive implications on the OEM / Legal Manufacturer relationship. For example, it is no longer sufficient to refer to the original OEM manufacturer's certificates within one’s own conformity assessment. Instead, the Legal Manufacturer now has to assess the documentation or make an assessment of the conformity of the product.
Furthermore, the Legal Manufacturer has to demonstrate the assessment to their NB and therefore needs to be prepared to submit the OEM's part of the technical documentation - including parts containing the OEM's trade secrets!
This constitutes a precarious dilemma since there is an obvious risk in handing over trade secrets to a party (i.e. the Legal Manufacturer) with an obvious incentive to make use of such secrets."
Consequently, MedtechVault is built to handle this situation. Information access can be strictly regulated and access to applicable documents can be handed out on a need-to-know basis. In this way, the OEM's documentation is available to the NB within the scope of the assessment of the Legal Manufacturer but cannot be accessed by the Legal Manufacturer himself."
So what are the technical prerequisites required to start working with MedtechVault?
"Next to none. You upload your technical documentation onto a hosted digital platform. The system is compatible with documents of any file format. MedtechVault operates as a seamless add-on to any ERP and QMS already in place. The data is stored on highly secured servers located here in Europe. This means zero capital expenditures, zero maintenance, and zero IT overhead for our customers."
In your opinion, who will benefit from MedtechVault?
"Anybody subjected to a conformity assessment process will benefit from MedtechVault, especially Legal Manufacturers working with OEM manufacturers. MedtechVault will speed up the communication, free up valuable QARA resources and accelerate your assessment procedure."