The IMDRF terminologies - a common risk language
Have you ever struggled to describe Hazardous Situations so it was clear to all stakeholders what you intended to say?
Did you spend a lot of time to come up with a concisely written Sequence of Events and then the first person to review your document claims to not understand what you intended to convey?
When describing your harms, have you ever wished that someone had put together a list of all possible harms, so you could just pick the one, which is applicable for this particular situation?
And then after your product release, did a Risk occur that you did not foresee?
Common Terminology by curtesy of the IMDRF
If you have ever experienced one or more of the above, there might be some help out there. The International Medical Device Regulators Forum (http://www.imdrf.org) has created a document called IMDRF terminologies for categorized Adverse Event Reporting (AER): terms, terminology structure, and codes.
Although that is quite a mouthful, this document can make your life a lot easier. It provides an extensive list of possible medical device problems, possible harms, and related causes. Each term is assigned a code, which has to be used when creating a Manufacturer Incident Report as required by the MDR (https://ec.europa.eu/docsroom/documents/41681).
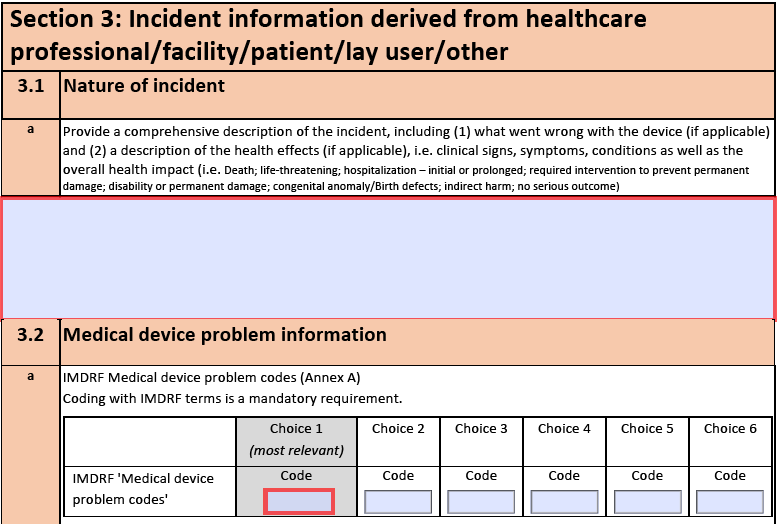
These codes can also be used when reporting Adverse Events to the FDA by means of a Medical Device Report (https://www.fda.gov/medical-devices/mandatory-reporting-requirements-manufacturers-importers-and-device-user-facilities/mdr-adverse-event-codes).
Is the terminology only applicable for post-market events?
Although the terms compiled by the IMDRF have a strong focus on Post Market incidents, they are also useful in your pre-market design risk assessments. When performing your ISO 14971 compliant Risk Analysis during the development phase, a lot of time is (and should be) spent on the risk identification process to make sure all potential risks have been assessed and addressed.
In practice, this requires writing down and assessing the hazardous situations, what causes, and subsequent harms that could possibly arise by using your product. However, these are essentially the same as in a post-market scenario. Using the lists provided by IMDRF can speed up this process significantly.
So how does this make things easier for me?
The IMDRF lists act as an acceleration vehicle for your Risk Analysis. By using and analysing these established terms, you will save a significant amount of time when documenting all possible hazardous situations, causes, and harms. At the same time, the likelihood of overlooking a particularly hazardous situation, cause, or harm is greatly reduced.
Furthermore, ambiguities are reduced by using and referring to an established set of risk terminologies. Thus, you reduce the risk that other stakeholders, not just your colleagues, but also the auditors, will not misunderstand your carefully constructed Risk Analysis.
Using the IMDRF terminology in Aligned Elements
The lists are applicable to Aligned Elements projects using Risk Assessments using the Preliminary Hazard Analysis method.
It is possible to import the IMDRF items directly into Aligned Elements by using four import packages which you can download here.
The extension consists of lists containing a Design Control type called “IMDRF Item”, which have the attributes “Code” and “Definition”.
When importing them, you will need to map the types to types that exist in your configuration.
Note that the lists contain a large number of items that may not all be applicable to your particular device.
A pre-assessment step of the list content is therefore recommended before applying them to production projects.
The following mappings should be done.
- “Annex A, Medical Device Problems” (469 items) should be mapped to a type that represents “Potential Hazards” in your configuration.
- “Annex D, Investigation Conclusions” (35 items) should be mapped to a type that represents “Causes” in your configuration.
- “Annex E, Health Effects - Clinical Signs and Symptoms or Conditions” (797 items) should be mapped to a type that represents “Harms” in your configuration.
- “Annex F, Health Effects - Health Impacts” (64 items) should be mapped to a type that represents “Harms” in your configuration.
Please do not hesitate to ask for assistance at