Aligned Elements—A System That Adapts to You, Not the Other Way Around
Do you make medical devices?
If yes, you’re likely familiar with the technical documentation challenges that come with the territory.
We provide an excellent system to help you overcome those challenges.
"But how do I know that such a system won’t disrupt my processes?"
Good question!
You are the expert on your product and your organization. You’ve invested considerable effort in refining your processes and templates to optimize your work. The last thing you need is a tool that forces you to rework everything.
Not Aligned Elements.
Where other tools impose limitations that require substantial changes to your QMS, Aligned Elements adapts to your needs. From the start, we recognized that each medical device manufacturer operates with a unique set of SOPs, processes, and templates, carefully developed to suit their specific requirements. That’s why we designed Aligned Elements to be flexible—so it can reflect your processes, use your document templates, and present your terms and notions in the UI.
This results in higher user acceptance, a lower learning curve, a shorter adoption process, and reduced implementation risks.
How Aligned Elements Adapts to Your Needs
Design Control Naming and Traceability
We begin by identifying all your Design Control types and their expected traceability. We then ensure that each type includes exactly the data specified in your SOPs and templates. This significantly accelerates adoption, as your users will immediately recognize familiar terminology and structures.
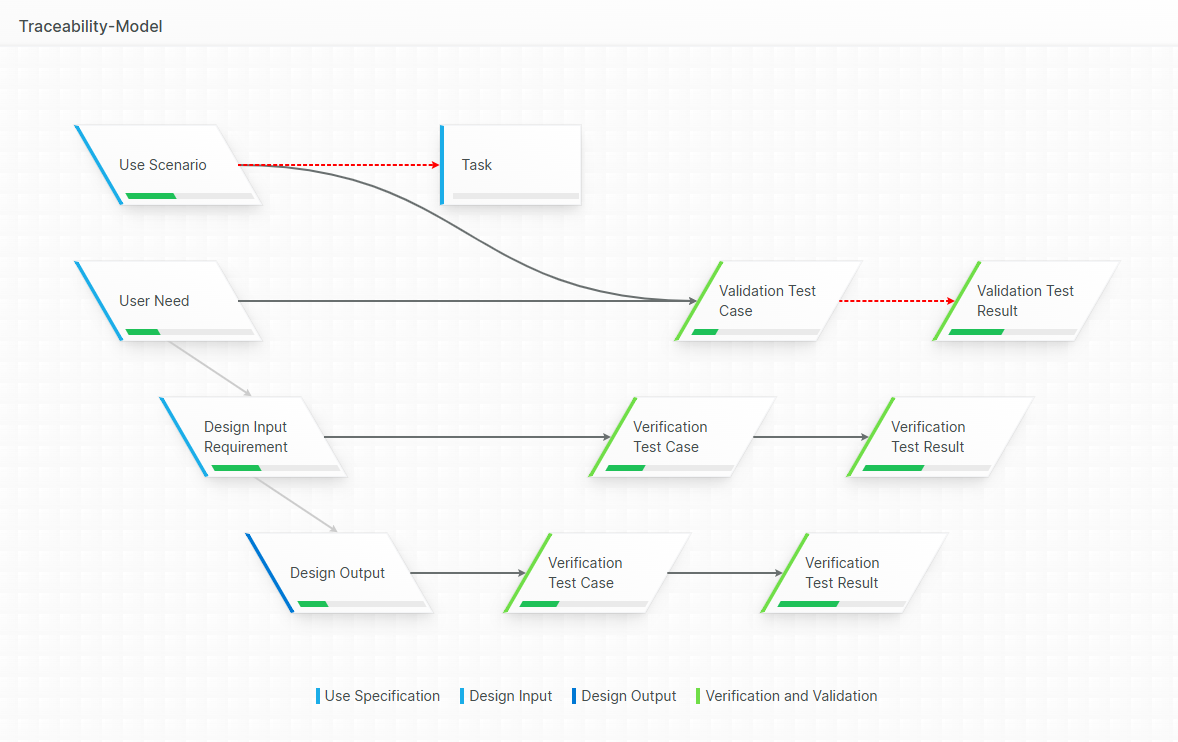
Trace Reporting
Our built-in Trace Table Designer allows you to create customized trace tables and matrices on the fly. Simply generate a trace table, inspect the results, modify the design, and rerun it within seconds. These traceability matrices can be exported into Word documents that maintain your unique corporate design.
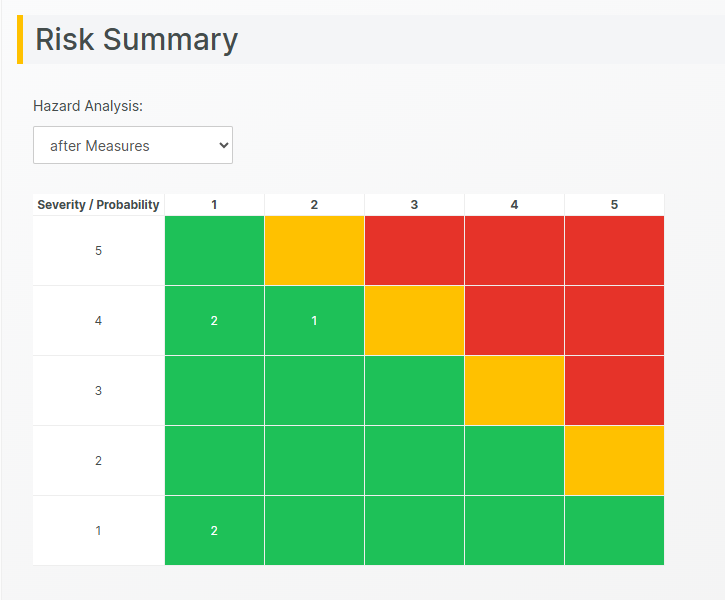
Tailored Risk Assessments
While ISO 14971 provides a standardized framework, we have found that risk assessments vary widely between organizations. Among the hundreds of medical device manufacturers we’ve worked with, each has had its own approach to risk assessment.
To accommodate this, we designed the Aligned Elements Risk Assessment section to be the most adaptable in the industry—and we continue to expand its capabilities.
Creating Technical Documents
Your QMS already includes tried-and-tested document templates—why not use them in Aligned Elements? Documents generated by Aligned Elements adopt the styling and structure of your existing templates while seamlessly integrating Design Control content.
You can mix and match structured data with anything created in MS Word, ensuring you get the best of both worlds. For maximum flexibility, Aligned Elements supports different Word styles for different contexts, such as formal styles for official documents and informal styles for internal reviews—giving you complete control.
Seamless Integration with Your Existing Toolset
Aligned Elements is explicitly process-independent, offering high flexibility in naming conventions and reporting formats to fit seamlessly into your workflow. It integrates with existing tools such as defect tracking systems (GIT, Atlassian Jira, Azure DevOps, Redmine, Trac, etc.), enhancing their capabilities with consistency checks, traceability, and reporting features.
What About Cost and Implementation Time?
We help every customer set up Aligned Elements according to their specific needs. This process typically takes only a few days. You can find pricing details [here].
Are You Forever Dependent on Our Expertise?
Absolutely not. As an Aligned Elements customer, you own your configuration. You can access and modify it at any time.
Thumbnail Designed by vectorjuice