Traceability in Medical Device Development
Traces are the glue.
Traces are the links that tie the development documentations together. They provide the basis for verifying completeness and consistency.
Traceability, the discipline of setting and managing traces, is a core activity in the medical device development process. Norms and directives such as ISO 13485, ISO 14971, IEC 62304, IEC 62366 and FDA CFR 21 QSR 820 explicitly prescribe it.
In essence, establishing traces between artefacts is a deceptively straightforward task. You just declare the trace and that's it. So why is traceability perceived as such as an annoying piece of work?
The main reason is change.
As mentioned above, a trace establishes a relation between dependent artefacts (e.g. a requirement and a specification). When change occurs to one party in this relationship (the requirement), the state of the traced artefact (the specification) may become invalid.
Word documents and Excel sheets are notoriously inept at handling this kind of problem since the artefacts are not aware of each other.
Traces in an Excel sheet are just dead text.
On the other hand, this is why traceability software tools such as Aligned Elements are so popular. Traceability software tools not only allow traces to be declared but are also able to track changes made to the traced artefacts.
A change in an artefact automatically highlights the affected traces and signalizes that they might have become invalid due to the change. In Aligned Elements, we call such a possibly invalidated relationship a "suspect trace".
The user now has the possibility to inspect the implicated artefacts and update them if needed. Or simply confirm that the change did not have any impact on traced artefacts. In Aligned Elements, an impact analysis entry is made in the audit trail as the user completes this action, in order to ensure accountability throughout the development process.
However, these gains are quickly lost if different artefact types (e.g. tests and risks) are kept in separate systems. Managing traces across system boundaries is often only possible by resorting to manual measures. Such as Excel. And then we are back to where we started.
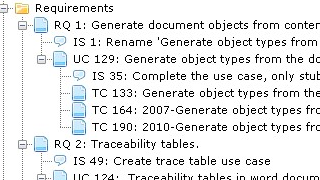
Keeping all traced artefacts in a single system further helps to disentangle a range of more mundane problems, such as finding out:
- If some artefacts have not been traced
- If some artefacts trace to artefacts of the wrong type
- If some traces simply don't make sense
In Aligned Elements, we have implemented traceability support following a few simple guidelines:
- Usability. It shall be easy to set or remove a trace, regardless of the working context. Therefore we have five different mechanisms for setting traces.
- Accountability. All trace-related operations shall be recorded chronologically in the audit trail.
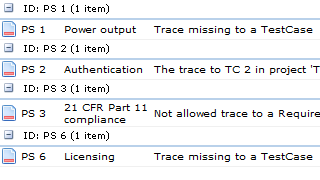
- Transparency. Missing, illegal and suspect traces must be immediately and automatically highlighted.
- Completeness. End-to-end traceability must be facilitated without having to cross-system boundaries.
- Reportability. Trace reports shall be customizable and configurable to match the quality requirements and look-and-feel criteria of each customer.
Again, traces are the glue.
Managed correctly with the right ALM support, they are (besides being regulatory necessities) a real asset to your design history file management. The traces help you ensure that completeness and consistency have been achieved throughout your development documentation. We are doing our best to make sure this becomes a smooth journey.