Design History File - Mind the Gap
"If it is not documented, then it has not been done", according to the FDA saying. "Documentation not available", or "Documentation not adequate" are the most frequently cited deviations in FDA Warning Letters. The effects of inconsistent documentation can be devastating, implying postponed market launches, product withdrawals, and fines.
The reason for this flood of FDA 483 warning letters, addressing seemingly obvious and simple errors, is not that the medical device manufacturers are ignorant or incompetent.
It is simply hard to keep the DHF documentation consistent.
The documentation requirements are many and detailed, the development projects often span over a long time-period, involves a large number of alternating team members, all contributing to the large set of deliverables that make up the Design History File/Technical File. The deliverables are highly interdependent and a small change can cause unexpected ripple-effects over large parts of the documentation. A considerable amount of the total project effort is thus placed in the handling and management of the DHF.
Not long ago, I was contacted by a customer. He had recently taken over a project with the objective to take the product to market. After a brief introduction, he found the project to be in a miserable state. The project had switched project manager four times during the last four years, the team members were all new, knowledge about the documentation process was lacking.
In short, the situation was very opaque.
The project manager wanted help with assessing the current state of the development documentation and within 10 minutes, we could extract the following information from Aligned Elements:
- About 20 Requirements lacked traces to Specifications. They were all software-related and entered by the same person during a short time-span about two years ago.
- All Specifications had adequate Test Cases assigned.
- About 10 Test Cases had never been executed. None of these were functional Test Cases and they had all been entered after the last milestone.
- About 10 Test Cases had the current state "Failed". Most of them had to do with reliability, maintenance, spare-parts, and life-time tests.
- About 15 Risks were insufficiently mitigated.
- About 10 Mitigations were not verified or implemented.
- All Word documents were up to date.
The project manager finally felt that he had some grip on the situation. He now had concrete errors to fix and also the names of the people to contact to get detailed information about each individual inconsistency.
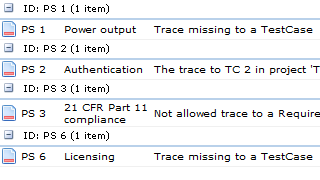
Aligned Elements addresses the issue of incompleteness with a range of integrated and automatic consistency and control functions. Aligned Elements is able to:
- In real-time, highlight any gaps and inconsistencies on any content set in the project.
- Provide reports that present a clear overview of the current consistency state of the project.
- Guide the user through a predefined process path to make sure errors are not created in the first place.
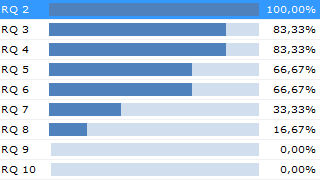
By the application of configurable validation rules, real-time checks can be executed on the documentation continuously. Faults and gaps are uncovered well in time before the auditor arrives or before the documentation is submitted to the notified body.
Knowing the state of the development documentation is invaluable for a medical device manufacturer. The list of open errors, representing the project’s “Documentation Quality Debt”, is an excellent estimator for how much work remains until the documentation is ready for release.
Reducing project risk by making the current documentation state transparent is an excellent way to increase the chances of a successful product launch.
Schedule a live demo and let us show you how Aligned Elements keeps your DHF complete and consistent